
Clinical Trial in India: Overcoming Regulatory Barriers to Innovation
Clinical trials are critical in developing new drugs, medical devices, and therapeutic regimens. They assist in determining the safety and efficacy of new interventions before their general distribution to the population. India is another country that has witnessed a surge in clinical trial activity, not least because of its vast and diverse population of patients, skilled healthcare professionals, and relatively low costs. However, many regulatory issues are impeding the industry’s full potential. The article reviews the key regulatory challenges faced in clinical trials in India and analyses their implications for stakeholders.
Regulatory Framework in India for Clinical Trials
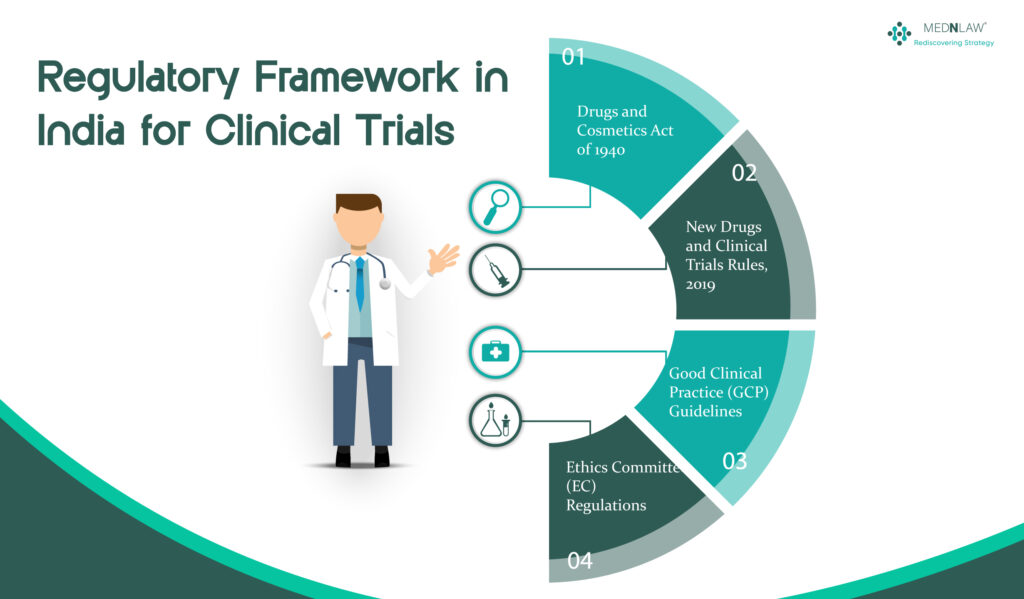
India’s regulatory framework for clinical trials is primarily governed by the Drugs and Cosmetics Act of 1940 and the rules made thereunder. The primary regulatory authority responsible for monitoring clinical trials is the CDSCO (Central Drugs Standard Control Organization) under the Ministry of Health and Family Welfare.
Several other regulations and guidelines are also instrumental in shaping the clinical trial landscape in India:
- New Drugs and Clinical Trials Rules, 2019: These set forth the guidelines and standards for drug development and clinical trials in India to ensure safety and efficacy, including procedures for approvals, conduct, and monitoring of trials and drug use.
- Good Clinical Practice (GCP) Guidelines: These are global guidelines for the planning, implementation, documentation, and publication of clinical trials, with a strong emphasis on data integrity and participant safety.
- Ethics Committee (EC) Regulations: These include regulations regarding the establishment and functioning of ethics committees (EC), which review and approve clinical trials to protect participants’ rights, safety, and well-being.
- Indian Council of Medical Research (ICMR): The ICMR has set forth its guidelines for ethical and scientific research practice in biomedical research in India, which allow for responsible research while also addressing Indian health concerns.
Key Regulatory Challenges
Ethical and Informed Consent Concerns
A significant challenge is making sure participants give informed consent. Many subjects (especially subjects from the rural regions of India) either have low literacy rates or lack understanding of medical terminologies. There are concerns about the poor participant knowledge of trial risks and benefits. Further, it also addresses the language variations that prevent participants from fully understanding consent forms. Pressure or undue influence, where the economically disadvantaged tend to be trial participants preoccupied with the financial incentives associated with trial participation rather than adversarial towards the risks.
Tight and Lengthy Approval Processes
Clinical trials in India have been criticised for their excessively long and tedious approval system. The New Drugs and Clinical Trials Rules 2019 sought to ease the process, but several challenges persist. There are several tiers of approval, such as CDSCO (Central Drugs Standard Control Organization), Ethics Committees, and other regulatory bodies. Additionally, inconsistency with the timelines causes clinical trials to be launched late, resulting in random outcomes with no transparency.
Frequent Regulatory Changes
Frequent changes in clinical trial regulation have created uncertainty among stakeholders in India. These changes result in more regulatory burdens for pharma and CROs. There is confusion around protocol adherence, as the introduction of the new guidelines conflicts with the old. The frequent changes in the regulations also lead to the reluctance of global sponsors to invest in clinical trials.
Ethics Committee Approval and Oversight
Ethics Committees are an essential safeguard for the rights and welfare of trial participants. However, challenges include the inhibitory function of Ethics Committees varying across departments, with some lacking appropriate expertise, ethical lapses in some trials due to inconsistent enforcement of moral standards. Further, delays in getting Ethics Committee approvals would make it take longer to start a trial.
Compensation Reporting and Safety Reporting Issues
Some of the significant changes for participants’ protection concerning the compensation for trial-related injury or death, wherein the Indian regulatory framework has also made it mandatory for compensation for trial-related injury or death. However, issues persist:
Disputes over compensation claims owing to ambiguity in the impact on allegations related to the definition of trial-related injury. Also, delayed or insufficient compensation due to bureaucratic hurdles. Additionally, underreporting of adverse events also changes safety data integrity.
Lack of Infrastructure and Capacity Constraints
Although India is a major destination for global clinical research, several gaps exist in both infrastructure and capacity building. This also means that few clinical trial sites have adequate facilities. The lack of a skilled clinical research workforce leads to poor trial quality.
Most of these records are in paper form, and there is a lack of monitoring (including in registration) of systems in compliance with international standards.
Lack of Public Trust and Perception Problem
Multiple historical controversies concerning unethical clinical trials have resulted in public distrust. The lack of transparency in trial procedures, which in turn results in media negativity, spurs public apathy. The Patients, particularly from marginalised communities, distrust following prior exploitation.
Concerns about IPR and Data Exclusivity
Intellectual property and data exclusivity concerns for pharmaceuticals conducting clinical trials in India, and the weak patent laws disincentivise investment. The absence of well-defined policy on data exclusivity makes India less attractive for conducting early-phase trials.
Cross-Border Compliance and Harmonization Challenges
India needs a regulatory framework on par with those of the rest of the world, such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). The challenges include inconsistency in adherence to Good Clinical Practice (GCP) standards and the adoption of international best practices. Further, the time spent obtaining approvals to run global multi-centre trials depends on different regulations.
Recent Reforms and Initiatives
In response to these challenges, the Indian government has implemented several reforms:
- New Drugs and Clinical Trials Rules, 2019: This set of rules helps with quicker approvals, shorter trial durations, and more clarity in compensation mechanisms.
- Telephonic New Entrepreneurship Development Scheme: The scheme empowers first-generation entrepreneurs to get loans.
- Strengthening Ethics Committees: Better rules and accreditation of ethics committees help build strong ethics and integrity within the team.
- Capacity Building Initiatives: Such programs help clinical research professionals enhance trial quality and provide better results.
How to Address Roadblocks to Regulatory Success
The following recommendations may promote India as a global clinical trial hub:
Better Informed Consent Processes
The informed consent form provides trial information in local languages through audiovisual aids. Third-party verification is required to guarantee that informed consent is genuinely given. Informed consent enhances patient education campaigns to promote awareness.
Simplification for Regulatory Approvals
It is crucial to make approval timelines fixed to improve predictability.
This further improves the harmonisation of approvals between CDSCO (Central Drugs Standard Control Organization) and Ethics Committees. Implement risk-based regulatory frameworks for low-risk trials.
Making Sure That Regulations Are Transparent and Stable
Liberalise foreign investment in key sectors to attract investments
Prior to bringing new regulations into effect, carry out regular stakeholder consultations. Implement clear guidelines to clear out any ambiguities.
Enhancing Ethics Committee Oversight
It is essential to train ethics committees according to a standardised model. Additionally, a national accreditation body for Ethics Committees should be implemented to ensure quality assurance. Periodic audits are also required to ensure compliance.
Enhancing Mechanisms for Reporting Compensation and Safety
Refining definitions related to trial injuries to limit disputes ensures timely compensation disbursement. The use of digital platforms to report adverse events in real time.
Investing in People and Infrastructure
More accredited trial sites with modern amenities are required. Further, funding for clinical research training programs also encourages the growth of public-private partnerships to enhance infrastructure.
Enhancing Public Trust
Running a community engagement program to create awareness.
It encourages open reporting of trial summaries and increases penalties to deter malpractice.
Conclusion
India has enormous potential when it comes to conducting clinical trials, but there are several regulatory challenges that need to be overcome to make the country a preferred destination for global research. India can create a strong and dependable clinical trial ecosystem that aids both the pharmaceutical sector and patients by introducing more transparency, simplified approvals, ethical compliance, and infrastructure development. That said, sustainable regulatory reforms, along with global collaboration, will be key in determining the future of clinical trials in India.





